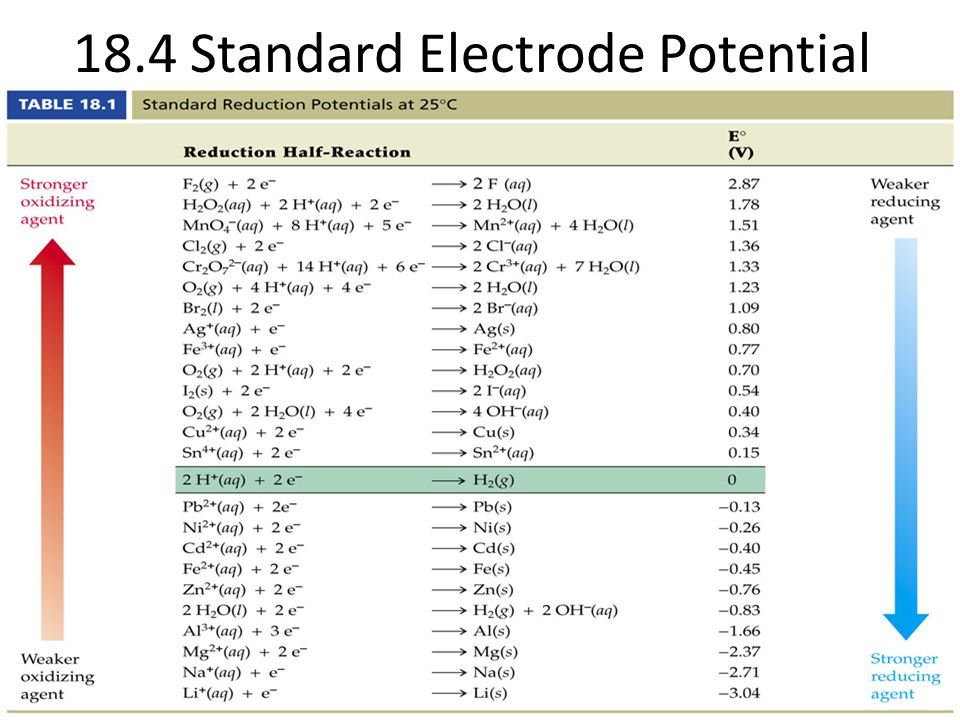
Standard Reduction Potential Example . — assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. — the reduction potential of a molecule under standard conditions (1 atm pressure, 1 m concentration, and 298 k. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. Use standard reduction potentials to determine the. For example, for the cell. — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at.
from ar.inspiredpencil.com
For example, for the cell. — the reduction potential of a molecule under standard conditions (1 atm pressure, 1 m concentration, and 298 k. — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. — assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. Use standard reduction potentials to determine the.
Standard Reduction Potential Table
Standard Reduction Potential Example For example, for the cell. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. Use standard reduction potentials to determine the. — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. For example, for the cell. — assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. — the reduction potential of a molecule under standard conditions (1 atm pressure, 1 m concentration, and 298 k. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Example — assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. For example, for the cell. — the reduction potential of a molecule under standard conditions (1 atm pressure, 1. Standard Reduction Potential Example.
From ecampusontario.pressbooks.pub
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential Example — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. For example, for the cell. — assigning the potential of the standard hydrogen. Standard Reduction Potential Example.
From www.youtube.com
Standard reduction potentials Redox reactions and electrochemistry Standard Reduction Potential Example — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. For example, for the cell. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. — assigning the potential of the standard hydrogen electrode (she) as. Standard Reduction Potential Example.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Standard Reduction Potential Example — assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. Use standard reduction potentials to determine the. once determined, standard reduction potentials can be used to. Standard Reduction Potential Example.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Example — assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. For example, for the cell. — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Use standard reduction potentials to determine the. — the reduction potential of a. Standard Reduction Potential Example.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential Example For example, for the cell. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. — assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. once determined, standard reduction potentials can be used to determine the. Standard Reduction Potential Example.
From f15.beauty
Standard Reduction Potential Table Standard Reduction Potential Example Use standard reduction potentials to determine the. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the standard reduction potential can be determined by subtracting. Standard Reduction Potential Example.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Example — assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. For example, for the cell. — the reduction potential of a molecule under standard conditions (1 atm pressure, 1. Standard Reduction Potential Example.
From ch302.cm.utexas.edu
Standard Reduction Potentials Standard Reduction Potential Example once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. — the reduction potential of a molecule under standard conditions (1 atm pressure, 1. Standard Reduction Potential Example.
From chem.libretexts.org
17.3 Standard Reduction Potentials Chemistry LibreTexts Standard Reduction Potential Example For example, for the cell. — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. — the reduction potential of a molecule under standard conditions (1. Standard Reduction Potential Example.
From www.youtube.com
Standard Reduction Potential YouTube Standard Reduction Potential Example For example, for the cell. — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. Use standard reduction potentials to determine the. the standard reduction potential. Standard Reduction Potential Example.
From www.slideserve.com
PPT STANDARD REDUCTION POTENTIAL PowerPoint Presentation, free Standard Reduction Potential Example Use standard reduction potentials to determine the. — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. For example, for the cell. the standard reduction potential. Standard Reduction Potential Example.
From www.youtube.com
The standard reduction potential for `Cu^(2+)Cu` is `+0.34V Standard Reduction Potential Example once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. — the reduction potential of a molecule under standard conditions (1 atm pressure, 1. Standard Reduction Potential Example.
From www.thetechedvocate.org
How to Calculate Standard Reduction Potential The Tech Edvocate Standard Reduction Potential Example — assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for. Standard Reduction Potential Example.
From www.scribd.com
Standard Reduction Potentials Data Extended pdf Standard Reduction Potential Example For example, for the cell. once determined, standard reduction potentials can be used to determine the standard cell potential, e ∘ cell, for any cell. — the reduction potential of a molecule under standard conditions (1 atm pressure, 1 m concentration, and 298 k. Use standard reduction potentials to determine the. — assigning the potential of the. Standard Reduction Potential Example.
From pveducation.org
Standard Potential PVEducation Standard Reduction Potential Example — the reduction potential of a molecule under standard conditions (1 atm pressure, 1 m concentration, and 298 k. — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. — assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of. Standard Reduction Potential Example.
From www.slideserve.com
PPT Chapter 18 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Example — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Use standard reduction potentials to determine the. — the reduction potential of a molecule under standard conditions (1 atm pressure, 1 m concentration, and 298 k. the standard reduction potential can be determined by subtracting the. Standard Reduction Potential Example.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Example — the standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. the standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard. For example, for the cell. — assigning the potential of the standard hydrogen. Standard Reduction Potential Example.